ShanDong Look Chemical Co.,Ltd

4'-Methylacetophenone CAS 122-00-9 | Look Chemical
CAS No. 122-00-9
EINECS: 204-514-8
Chemical Name: 4′-Methylacetophenone
Category: Pigments and dyes; Food additives; Flavors and fragrances
Molecular Formula: C9H10O
Molecular Weight: 134.18
Purity: 99%min
Brand Name: Look Chemical
Place of Origin: China
Name: 4′-Methylacetophenone
CAS: 122-00-9
Purity: 99%min
MOQ: 1KG
The Complete Guide of 4'-Methylacetophenone
4'-Methylacetophenone Chemical Formula
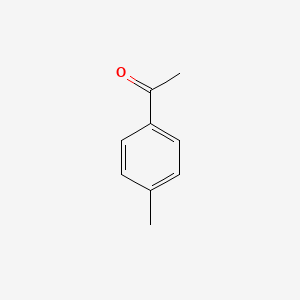
Basic Info
| CAS No: | 122-00-9 |
| MF: | C9H10O |
| Molecular Weight: | 134.18 |
| EINECS: | 204-514-8 |
| Appearance: | Colorless liquid |
| Product Name: | 4′-Methylacetophenone |
| Other Name: | Acetophenone, 4′-methyl-; p-Methoxyacetophenone |
| Place of Origin: | China |
| Brand: | Look Chemical |
What is 4'-Methylacetophenone?
4′-Methylacetophenone has a fruity, floral, and sweet taste similar to acetophenone, as well as a strawberry flavor. It can be prepared by slowly adding a mixture of ethylene chloride and ALCL under ice bath and vacuum, keeping the temperature at+5 ° C and then increasing it to+20 ° C.
4′-Methylacetophenone has been identified in Brazilian rosewood oil and pepper. It is a colorless crystal with a milder floral aroma and sweetness than acetophenone. 4′-Methylacetophenone is prepared by the Friedel Craftsford reaction of toluene and acetic anhydride or acetyl chloride. It is used for the floral fragrance of mimosa and hawthorn perfume, especially soap perfume.
According to reports, essential oils distilled from the wood of Myrocarpus Fastigiatus, Myrocarpus Fronto Sus, Bois de Rose. It also reported on sour cherries, orange and grapefruit peel oil, blackcurrant, melon tiles, peaches, blackberries, celery, potatoes, tomatoes, cereal, pepper, cilantro, smoked fish, cognac, Parmesan cheese, cocoa cheese, cocoa cheese, cocoa, mango, cauliflower, broccoli, rice bran, buckwheat, dried bonito, cherimoya, calabash nutmeg and frankincense leaf oil, cooked cabbage, and Mandarin fruit juice.
4'-Methylacetophenone Uses
4′-Methylacetophenone is a methylated acetophenone, which is used in cosmetics and perfume. It has been shown that the presence of 4 ‘- methyl vinyl ketone can accelerate the photopolymerization of methyl methacrylate.
4′-Methylacetophenone is used as a seasoning agent. In the presence of sulfur, it reacts with morpholine to form 4- (p-tolylthioacetyl) – morpholine. In addition, it is used as an intermediate in the production of active drugs, perfume and cosmetics.
4′-Methylacetophenone is an aromatic ketone. It is a metabolite found or produced in brewing yeast.
Can be used in types such as shy flowers, hawthorn flowers, acacia, black locust, primrose, sunflower, hyacinth, lilac, lily, wood fragrance, moss fragrance, etc. It can be used together with coumarin, anisaldehyde, and jasmonal in soap lavender, coriander, magnolia, and new cut grass types. It can be used in a small amount in almond, vanilla bean flavor edible essence, and also in a small amount in tobacco essence.
This product has a fragrance similar to that of Shanchazi flowers, with aromas such as purple alfalfa, honey, strawberries, and a sharp and sweet floral and fruity aroma. GB 2760 1996 stipulates that edible spices are allowed to be used. It is mainly used to prepare essence of almond, nut, cherry, Xiangzhuo and strawberry.
References
4′-Methylacetophenone – Pubchem
CN201610777734.X Preparation method of p-methylacetophenone
4'-Methylacetophenone for Sale
Application of 4'-Methylacetophenone
- Used to prepare celecoxib
Celecoxib, trade name: Celebrex, chemical name: 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazole-1-yl]benzene Sulfonamide, with the chemical formula C17H14F3N3O2S, is a specific type 2 cyclooxygenase (COX‑2) inhibitor launched by the American Searle Company. Its selectivity for COX‑2 is 375 times that of COX‑1 and is mainly used for bone For the treatment of arthritis and rheumatoid arthritis, its anti-inflammatory activity is equivalent to that of indomethacin, but it has almost no gastrointestinal side effects. The synthesis method of celecoxib mainly involves Claisen condensation of p-methylacetophenone to obtain β-diketone intermediate, and then cyclization with p-hydrazinobenzenesulfonamide to obtain celecoxib. - Used to synthesize 4-chloro-2-cyano-N,N-dimethyl-5-(4-methylphenyl)-1H-imidazole-1-sulfonamide
4-Chloro-2-cyano-N,N-dimethyl-5-(4-methylphenyl)-1H-imidazole-1-sulfonamide (cyanofenazole) was developed by Ishihara Sangyo Co., Ltd. of Japan. The new generation of imidazole fungicides jointly developed with BASF is mainly used to prevent and control diseases caused by Oomycete pathogens, such as potato late blight, tomato late blight, grape downy mildew, cucumber downy mildew, etc. It has good protective activity, long residual effect, and is resistant to rain erosion. It can be used for soil treatment or foliar spraying, and has very low toxicity and good environmental compatibility. Used to synthesize 4-acetylbenzoic acid
4-Acetylbenzoic acid is mainly used to synthesize the new drug intermediate N-methyl-2-(4-acetyl-5-nitrobenzimidazole), and benzimidazole and its derivatives are the active ingredients of many new drugs. Such as Cemazole antihistamines, etonazine, strong analgesics, benzylchloride plus imidazole, antispasmodics and antifungal drugs belong to this type of derivatives, and their synthesis has certain theoretical significance and strong practical value. Previously The production cost of synthesizing 4-acetyl benzoic acid is high, and the three wastes are discharged greatly, which greatly affects the use and promotion. In the existing technology, the reaction temperature is high, the dosage of potassium permanganate is large, and the reaction time is long.CN201210161777.7 provides a preparation method of 4-acetyl benzoic acid with low production cost, environmentally friendly production process and high yield. Includes the following steps:
(1) Oxidation, add p-methylacetophenone, water and anhydrous zinc chloride into the reaction pot, stir evenly, slowly heat up to 35~40℃, divide the potassium permanganate into five equal parts, every 15~20 Add one portion every minute, and control the reaction temperature to 48~55°C. After the addition, control the reaction temperature to 40~45°C, keep it warm for 1.5 hours, then lower the temperature to 17~22°C, centrifuge, and dry to obtain 4-acetylbenzene. Crude formic acid.
(2) Mix the prepared crude 4-acetyl benzoic acid with anhydrous acetic acid, heat and reflux for 0.5 to 1.5 hours, filter while hot, centrifuge, and dry to obtain 4-acetyl benzoic acid.
Learn More
Professional 4′-Methylacetophenone Supplier
As a professional chemical supplier, we have ten years of chemical supplier experience, more professional and reliable, 24 hours service. Provide free samples.If you want to know more, please come to consult us.
Service
Pre-Sales Service
* Prompt reply and 24 hours online, professional team to provide best price and high quality product.
* Sample testing support.
* Every batch of products will be tested to ensureits quality.
* The packing also can be according the customers` requirment.
* Any inquiries will be replied within 24 hours.
* We provide Commerical Invoice, Packing List, Bill of loading, COA , Health certificate and Origin certificate. If your markets have any special requirements, let us know.
After-Sales Service
* The fact of logistics information monitoring.
* Any questions about the product can be consulted at any time.
* Product has any problem can return.
1. ≤50kg, Express delivery recommended, usually called as DDU service;
2. ≤500kg, Air shipping recommended, usually called as FOB, CFR, or CIF service;
3. >500kg, sea shipping recommended, usually called as FOB, CFR, or CIF service;
4. For high value products, please select air shipping and express delivery for safe.
Do you accept sample order?
We will make samples before mass production, and after sample approved, we’ll begin mass production. Doing 100% inspection during production, then do random inspection before packing.
What’s your MOQ?
Our MOQ is 1kg. But usually we accept less quantity such as 100g on the condition that sample charge is 100% paid.
Is there a discount?
Different quantity has different discount.
How to confirm the product quality before placing orders??
You can get free samples for some products,you only need to pay the shipping cost or arrange a courier to us and take the samples. You can send us your product specifications and requests,we will manufacture the products according to your requests.- Do you supply product report?Yes. We’ll give you product analysis report before shipping.
Packaging and Shipping of 4'-Methylacetophenone







