
Name: Vanadium(iv) oxide
Cas: 12036-21-4
Purity: 99%
MOQ: 1KG
Directory Guidance on Vanadium(iv) oxide
Chemical Structure
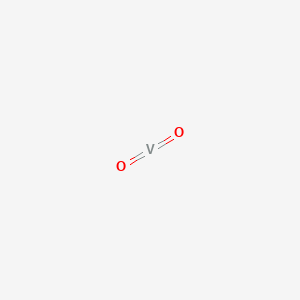
Basic Info
Item description of Vanadium(iv) oxide
The chemical formula of Vanadium(iv) oxide is VO2. Molecular weight 82.94. Dark blue grainy crystal. Specific gravity 4.339. Melting point 1967 ℃. Flammable. It comes from the configuration of sodium chloride. Insoluble in water, soluble in acid and antacid.
Having both sexes: Vanadium(iv) oxide liquifies in acid to form a tetravalent vanadyl ion (VO2+); Liquify in alkali to create vanadate salts (M2V4O9 and M2V2O5). The chemical residential or commercial properties are not really steady. Gradually oxidizes in air and responds with nitric acid to form vanadium pentoxide.
Vanadium(iv) oxide is prepared by heating vanadium pentoxide and vanadium trioxide in equimolar ratios alone from air or melting vanadium pentoxide with oxalic acid, carbon, phosphorus, and sulfur dioxide. Vanadium(iv) oxide is primarily utilized as a high-temperature driver.
Residence of Vanadium(iv) oxide
Black to dark blue tetragonal crystal, rutile crystal kind. Stable in air at area temperature. Family member thickness 4.339, melting point 1967 ℃. Moisture taking in, insoluble in water, soluble in acid and alkali.
Product Introduction
| Vanadium(iv) oxide Chemical Properties |
| Melting point | 1967°C | ||||||||||||||
| density | 4.339 g/mL at 25 °C(lit.) | ||||||||||||||
| solubility | insoluble in H2O; soluble in acid solutions, alkaline solutions | ||||||||||||||
| form | Powder | ||||||||||||||
| Specific Gravity | 4.339 | ||||||||||||||
| color | black | ||||||||||||||
| Water Solubility | Insoluble in water. | ||||||||||||||
| Crystal Structure | MoO2 type | ||||||||||||||
| crystal system | Monoclinic | ||||||||||||||
| Space group | P21/c | ||||||||||||||
| Lattice constant |
| ||||||||||||||
| Exposure limits | NIOSH: Ceiling 0.05 mg/m3 | ||||||||||||||
| CAS DataBase Reference | 12036-21-4(CAS DataBase Reference) | ||||||||||||||
| EPA Substance Registry System | Vanadium oxide (VO2) (12036-21-4) |
Product Name | Vanadium(iv) oxide |
brand | China Lookchemical |
Specification | 99% |
MOQ | 1KG |
Storage conditions | Store in cool & dry place, Keep away from strong light and heat. |
Sample | Available |
Product Packaging

Vanadium(iv) oxide is a binary oxide with a solid correlated electron system. Because of the strong interactions between its internal electrons, orbitals, lattice, and rotate, changes in local electrons and crystal structure caused by tiny outside stimuli can generate relatively easy to fix steel insulator stage changes in Vanadium(iv) oxide. With stage shift, the crystal structure, resistance, infrared transmittance, refractive index, and magnetization of Vanadium(iv) oxide undergo fast changes. It is exactly because of the stage change temperature level of Vanadium(iv) oxide near area temperature and the sudden changes in its physical and chemical properties prior to and after the phase change that it has a really wide range of application prospects in electronics, armed forces, every day life, and other fields.
Vanadium(iv) oxide is a suitable option to replace silicon products for the new generation of low-power digital gadgets as a result of its one-of-a-kind residential or commercial properties. Swiss researchers lately showed making use of Vanadium(iv) oxide in aerospace interaction systems to accomplish programmable RF electronic functionality, highlighting the prospective applications of this oxide.
characteristic
Vanadium(iv) oxide displays phase change qualities, with a morphology that can be changed between an insulator and a steel. It acts as an insulator at space temperature level and as a steel conductor above 68 degrees Celsius. This is due to the fact that its atomic structure can change from an area temperature level crystal framework to a metal framework at temperature levels above 68 levels Celsius, and the makeover occurs in less than 1 millisecond, which is a benefit for electronic applications. Associated study has actually led lots of people to think that Vanadium(iv) oxide might come to be an innovative material for the future electronics sector.
application
For several years, scientists have examined the phase change behavior of Vanadium(iv) oxide under various outside stimulation conditions and associated device applications. Details stage shift excitation resources consist of warmth, light, electricity, electrochemistry, magnetism, stress, etc. The stage shift procedure and applications of Vanadium(iv) oxide under various excitement problems vary greatly. Starting from the phase shift of Vanadium(iv) oxide and the special physical and chemical properties triggered by phase transition, this article summarizes the device of Vanadium(iv) oxide phase shift under different stimuli such as warmth, light, electricity, electrochemistry, stress, and magnetic field, along with the response and application of Vanadium(iv) oxide gadgets under matching stimulation conditions.
Related References
Wikipedia-Vanadium(iv) oxide
Manufacturer of Vanadium(iv) oxide
China Lookchemical is based in Shandong, serves the whole country, faces the world, and is determined to become an excellent supplier and service provider in the chemical industry.
As a top manufacturer of surfactant,cosmetic raw materials, disinfectants, flavors, and fragrances in China, we are committed to producing high-quality products and have won high praise from customers all over the world.
In the next 10 years or even longer, Lookchemical will always adhere to the tenet of quality first and reputation first, and devote itself to providing customers with thoughtful and perfect services.
As a professional Vanadium(iv) oxide manufacturer, Look Chemical has served more than 6,000 customers around the world. Because of our high-quality products and professional services, we have won high praise from most of our customers.
Pre-Sales Service
*The company cooperates with research institutes. We strictly control the process of raw materials up to the finished product.
*Dedicated to serving customers first, we provide reasonable prices, high quality products and timely delivery and follow-up services.
* Timely reply and 24 hours online, the professional team will provide you with the most favorable prices and high-quality products.
* The sample supports testing and inspection.
* Each batch of products will be tested to ensure that its quality meets user needs.
*Packaging can also be made according to customer requirements.
*Any inquiries will be answered by our relevant personnel within 24 hours.
*We will provide you with commercial invoice, packing list, packing list, COA, health certificate and certificate of origin if you need it. If your market has other special requirements, please let us know.
After-Sales Service
*We will monitor the logistics information in real time and will share the information with you.
* You can consult us at any time if you have any questions about the product, and we will answer you in time.
*If you have any questions about the product, you can report it to us, we will deal with it in time for you, and the product can be returned.
To ensure the convenience of clients,we always set accurate reply for crucial question.Clients will get answer by inputting “keywords”to help clients solve relevant questions.
DO YOU ACCEPT SAMPLE ORDER?
We will make samples before mass production. After the samples are approved by the department and users, we will start mass production. During the production process, we will carry out 100% inspection, and then carry out random inspection before packaging.
WHAT’S YOUR MOQ?
Our MOQ is 1kg. But usually we can also accept a smaller quantity, such as 100g, if you need it, provided that the sample fee is 100% paid.
IS THERE A DISCOUNT?
Different quantity has different discount.
HOW TO CONFIRM WHETHER THE PRODUCT QUALITY MEETS OUR NEEDS BEFORE PLACING AN ORDER?
You can consult us to get free samples of some products, you only need to pay the freight or arrange express delivery to us and get the samples. You can send us the product specifications and requirements you need, and we will manufacture products according to your requirements.
DO YOU SUPPLY PRODUCT REPORT?
Yes. We’ll give you product analysis report before shipping.
LEARN MORE ABOUT US
Shandong Look Chemical
1. For products ≤50kg, we recommend using express delivery, which is usually called DDU service (discounted, convenient).
2. For products ≤500kg, we generally recommend air freight, which is usually called FOB, CFR or CIF service (fast and efficient).
3. For products >500kg, we generally recommend shipping by sea, which is usually called FOB, CFR or CIF service (economical, safe).
4. For high-value products, please choose air or express to ensure the safety of product transportation.
Company Info
At the same time, we can also provide customers with excellent transportation services. Adhere to customer centricity and integrity as the source of development, and gradually improve the company’s various systems. We are high-quality management personnel who have been engaged in chemical trade for many years, with a modern market management philosophy, and constantly explore customer market demands using flexible methods.

Cooperation Partner
Hot Products


Distributor Keratin hydrolyzed CAS 69430-36-0 | China Lookchemical

Wholesale Vanadium(iv) oxide CAS 12036-21-4 | China Lookchemical

Factory Sodium cocoyl glutamate CAS 68187-32-6 | China Lookchemical

Free Sample Ethyl silicate CAS 78-10-4 | China Lookchemical

